鍑皬瀹氭爱浠?铔嬬櫧璐ㄦ祴瀹氫华)锛屾槸鏍规嵁鍑皬瀹氭爱娉曡璁$殑鑷姩鍖栨祴姘捀棣忕郴缁燂紝閫傜敤浜庣伯娌规娴嬨€侀ゲ鏂欏垎鏋愩€佹鐗╁吇鍒嗘祴璇曘€佸湡鑲ユ娴嬨€佺幆淇濄€佸尰鑽€佸寲宸ョ瓑琛屼笟鐨勫垎鏋愩€佹暀瀛﹀強鐮旂┒涓富瑕佺敤鏉ユ娴嬬伯椋熴€侀鍝併€佷钩鍒跺搧銆侀ギ鏂欍€侀ゲ鏂欍€佸湡澹ゃ€佹按銆佽嵂鐗┿€佹矇娣€鐗╁拰鍖栧鍝佺瓑涓殑姘ㄦ爱銆佽泲鐧借川姘瓑鍚噺銆?nbsp;
鍑皬瀹氭爱娉曟槸娴嬪畾鍖栧悎鐗╂垨娣峰悎鐗╀腑鎬绘爱閲忕殑涓€绉嶆柟娉曘€傚湪鏈夊偓鍖栧墏鐨勬潯浠朵笅锛岀敤娴撶~閰告秷鍖栨牱鍝佸皢鏈夋満姘兘杞彉鎴愭棤鏈洪摰鐩愶紝鐒跺悗鍦ㄧ⒈鍖栬捀棣忓皢閾电洂杞寲涓烘皑锛岄殢姘磋捀姘旇捀棣忓嚭鏉ュ悗涓鸿繃閲忕殑纭奸吀娑插惛鏀讹紝鍐嶄互纭吀鎴栫洂閰告爣鍑嗘憾娑叉淮瀹氾紝鏍规嵁閰哥殑娑堣€楅噺涔樹互鎹㈢畻绯绘暟(鍚爱閲徝?.25=铔嬬櫧鍚噺)锛屽苟鎹㈢畻鎴愯泲鐧借川鍚噺锛屽嵆鍙绠楀嚭鏍峰搧涓殑鍚爱閲忋€傛娉曟槸缁忓吀鐨勮泲鐧借川娴嬪畾鏂规硶銆傚嚡姘忓畾姘硶琚畾涓洪鍝佷腑铔嬬櫧璐ㄥ惈閲忔祴瀹氱殑鐜拌鍥藉鏍囧噯鍙婂浗闄呴€氳鐨勬祴瀹氭柟娉曘€侟br/>鍙嶅簲鏂圭▼寮忥細
1.鏈夋満鐗╀腑鐨勮兒鏍瑰湪寮虹儹鍜 CuSO4锛屾祿 H2SO4浣滅敤涓嬶紝纭濆寲鐢熸垚(NH4)2SO4锛屽弽搴斿紡涓猴細
2NH2+ H2SO4+ 2H = (NH4)2SO4(鍏朵腑CuSO4鍋氬偓鍖栧墏)
2.鍦ㄥ嚡姘忓畾姘櫒涓笌纰变綔鐢紝閫氳繃钂搁閲婃斁鍑 NH3锛屾敹闆嗕簬 H3BO3 婧舵恫涓紝鍙嶅簲寮忎负锛欬br/> (NH4)2SO4+ 2NaOH = 2NH3+ 2H2O + Na2SO4
2NH3+ 4H3BO3= (NH4)2B4O7+ 5H2O
3. 鐢ㄥ凡鐭ユ祿搴︾殑 H2SO4(鎴 HCl )鏍囧噯婧舵恫婊村畾锛屾牴鎹瓾CI娑堣€楃殑閲忚绠楀嚭姘殑鍚噺锛岀劧鍚庝箻浠ョ浉搴旂殑鎹㈢畻鍥犲瓙锛屾棦寰楄泲鐧借川鐨勫惈閲忥紝鍙嶅簲寮忎负锛欬br/> (NH4)2B4O7+ H2SO4+ 5H2O = (NH4)2SO4+ 4H3BO3
(NH4)2B4O7+ 2HCl + 5H2O = 2NH4Cl + 4H3BO3
璁$畻鍏紡锛欬/strong>
| X |
= |
(V1- V2) 脳 C 脳 0.014 |
脳 |
F |
脳 |
100 |
| m |
X锛氭牱鍝佽泲鐧借川鍚噺 (g/100g 鎴杇/100mL)
V1銆乂2锛氭祴瀹氱敤鏍枫€佽瘯鍓傜┖鐧芥秷鑰楃洂閰告爣鍑嗘憾娑茬殑浣撶Н (mL)
C锛氱洂閰告爣鍑嗘恫鐨勬懇灏旀祿搴 (mol/L)
0.014锛?mol/L鐩愰吀鏍囧噯娑?mL鐩稿綋浜庢爱鐨勫厠鏁?br/> m锛氭牱鍝佽川閲?g)鎴栦綋绉?mL)
F锛氳泲鐧借川鎹㈢畻绯绘暟6.25
鎹㈢畻绯绘暟锛欬br/>涓€鑸鐗?.25銆佷钩鍒跺搧6.38銆侀潰绮?.70銆侀珮绮变负6.24銆佽姳鐢熶负5.46銆佺背涓?.95銆佸ぇ璞嗗強鍏跺埗鍝佷负5.71銆佽倝鍒跺搧涓?.25銆佸ぇ楹﹀皬绫崇嚂楹︺€佽8楹︿负5.83銆佽姖楹讳笌鍚戞棩钁典负5.30銆侟br/>瀹為獙瑁呯疆锛欬/strong>
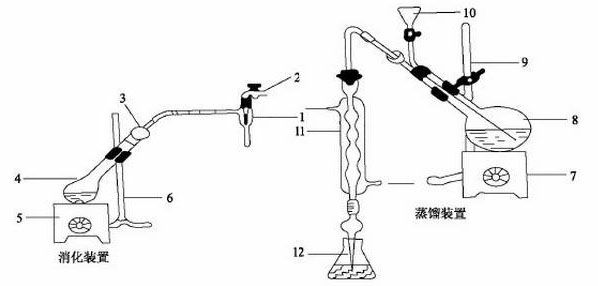
|
| 甯哥敤鍑皬瀹氭爱娑堝寲銆佽捀棣忚缃?br/>1鈥旀按鍔涙娊姘旂 2鈥旀按榫欏ご 3鈥斿€掔疆鐨勫共鐕ョ 4鈥斿嚡姘忕儳鐡?nbsp; 5銆?鈥旂數鐐堻br/>8鈥旇捀棣忕儳鐡?nbsp; 6銆?鈥旈搧鏀灦 10鈥旇繘鏍锋紡鏂?nbsp; 11鈥斿喎鍑濈 12鈥旀帴鏀剁摱 |
鎵€闇€璇曞墏锛欬br/>1銆佹秷鍖栨恫锛氱~閰伏br/> 2銆佸偓鍖栧墏锛氱~閰搁摐銆佺~閰搁捑
3銆佺⒈婧舵恫锛?0%姘㈡哀鍖栭挔
4銆佸惛鏀舵恫锛?%纭奸吀
5銆佹贩鍚堟寚绀哄墏锛?浠?.1%鐢插熀绾箼閱囨憾娑蹭笌5浠?.1%婧寸敳閰氱豢涔欓唶婧舵恫涓寸敤鏃舵贩鍚堛€備篃鍙敤2浠?.1%鐢插熀绾箼閱囨憾娑蹭笌1浠?.1%娆$敳鍩鸿摑涔欓唶婧舵恫涓寸敤鏃舵贩鍚堛€侟br/> 6銆佹爣鍑嗘淮瀹氭恫锛?.1mol/L鐩愰吀鏍囧噯婧舵恫銆侟/span>



